Revolutionizing Clinical Research
We eliminate the operational chaos of clinical trials by providing purpose-built tools that transform fragmented, manual processes, delivering real-time data visibility and control, behavioral incentives, and predictive analytics.
Key Features
Every component designed to empower patients, doctors, and monitors and accelerate trial outcomes
Complete Patient Visibility
Doctors gain instant, comprehensive access to each patient's health data, diary entries, and trial progress through automated data flows and real-time updates
Click to learn more
Unified Monitoring Dashboard
CRAs gain complete visibility across multiple trials with real-time metrics, compliance tracking, and automated alerts for protocol deviations
Click to learn more
Gamified Adherence System
Voucher-based reward system with achievement streaks transforms patient engagement, directly addressing the industry's adherence crisis
Click to learn more
Automated Anomaly Detection
Intelligent monitoring algorithms flag unusual patterns, adverse events, and protocol deviations instantly, enabling proactive intervention
Click to learn more
Seamless EDC Integration
Automated synchronization with global EDC platforms eliminates manual data reconciliation while maintaining enterprise-grade security and compliance
Click to learn more
Patient-Centric Mobile Platform
Comprehensive mobile experience for effortless daily logging, symptom reporting, medication tracking, and instant communication with clinical teams
Click to learn more
THE COST OF INEFFICIENCY IN CLINICAL TRIALS
Operational fragmentation creates cascading costs that delay life-saving treatments and inflate trial budgets
Each broken link compounds the problem, driving up costs and failure rates. But there's a solution.
Connecting the Missing Links
Medify addresses the operational fragmentation that delays clinical trials by unifying patient data collection, investigator workflows, and monitor oversight in one platform. Explore the custom-tailored solutions for each key stakeholder below.
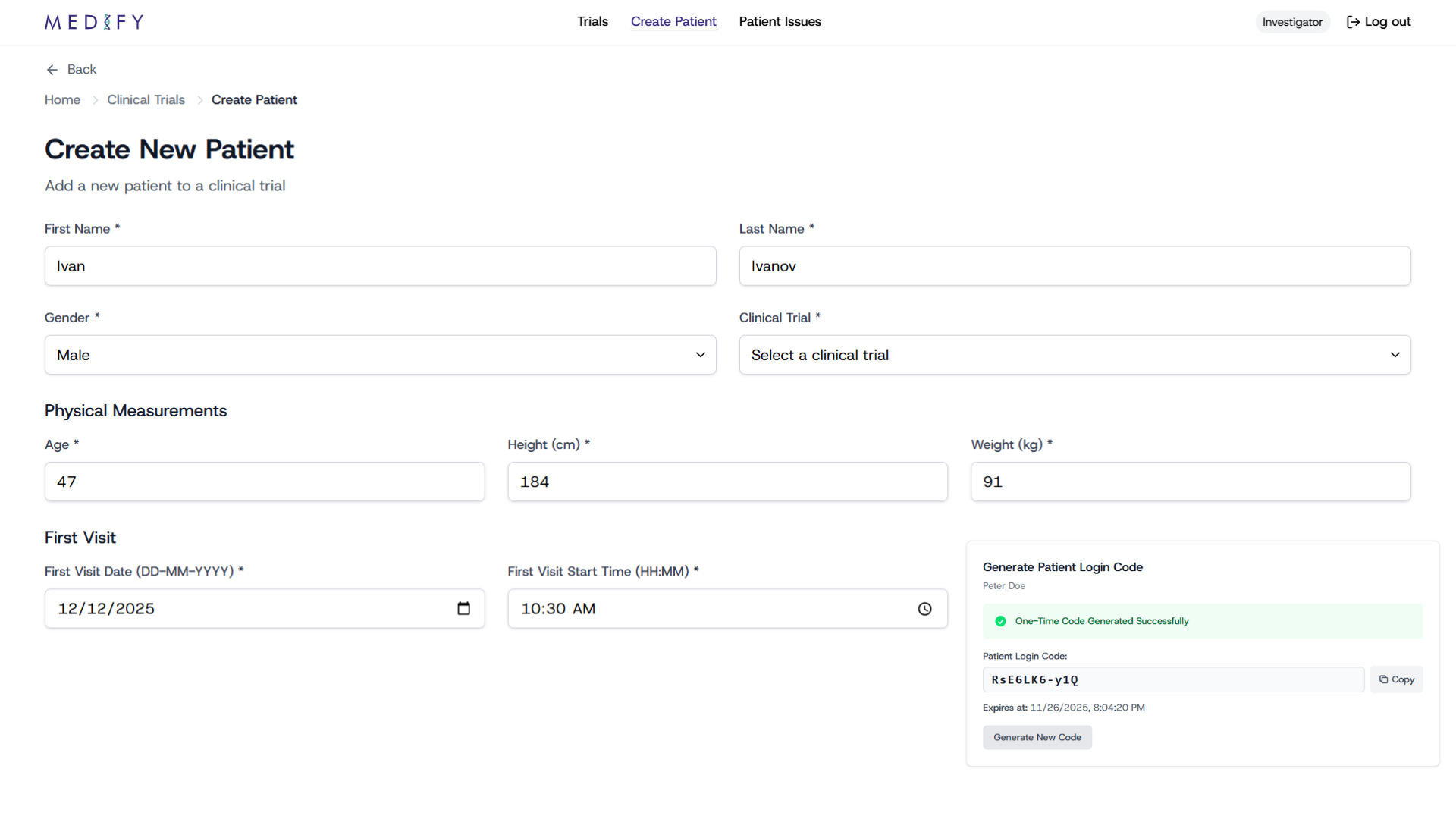
For Doctors
Instant Access & Streamlined Workflow
Medify gives doctors instant, secure access to each patient's data through automated data flows and built-in integrations with trial-critical systems.
Instantly enroll patients – one-time codes make onboarding fast and error-free
Complete visibility over each patient – view up-to-date health records and daily notebooks in one place
Automated alerts & notifications – be notified of adverse events or missing data so nothing critical goes unnoticed
Cross-systems integration – automatic syncing with global EDC platforms, which will eliminate copy-paste work
About Medify
Our Journey
Founded
Medify was created to address the fragmentation, low data qualirt, and outdated processes slow down clinical trials across Eastern Europe. We set out to build technology that finally closes these gaps.
Strategic Pivot
After industry interviews and early testing, we expanded beyond medication adherence and evolved into a unified clinical trial platform—aiming to solve workflow inefficiencies at scale, not just at the patient level.
Validated & Testing
Our direction was validated in JA Bulgaria’s Beyond Pre‑accelerator, and we are now testing our late‑stage MVP with real clinical sites. The platform includes proper European localization, offline-first capabilities, and end-to-end regulatory compliance.
Get in Touch
Ready to revolutionize your clinical trials? Let's talk about how Medify can help.
